IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 34 Issue 1 (January 2017)
Citation: Cuculescu-Santana, M, Horn, C, Briggs, RN, Bowe, C and Geraughty, ML (2017). Seasonal Changes in the Behaviour and Enclosure Use of Captive Asian Small Clawed Otters. IUCN Otter Spec. Group Bull. 34 (1): 29 - 50
Seasonal Changes in the Behaviour and Enclosure Use of Captive Asian Small Clawed Otters
Mirela Cuculescu-Santana1*,Chris Horn2,Rachel N. Briggs1, Charlotte Bowe and Megan L. Geraughty1
1 Department of Applied Sciences, Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne, NE1 8ST, UK
* Corresponding Author Email mirela.cuculescu@northumbria.ac.uk
2Blue Reef Aquarium, Grand Parade, Tynemouth, NE30 4JF, UK
     |
| ( Received 8th December 2016, accepted 25th January 2017 ) |
| Download PDF (984 KB) |
| Abstract: The influence of seasonal changes in temperature on the behaviour of tropical mammals kept in zoos and aquaria in temperate climate regions is very little studied. This article describes seasonal differences in the behavioural time budget and enclosure use of two male Asian small-clawed otters (Aonyx cinereus) held in an indoor enclosure at the Blue Reef Aquarium Tynemouth in the North-East of England (55°N). The otters studied spent significantly more time in the water in summer (water temperature 18-19°C) than in winter (water temperature 11-12°C). Swimming represented 33.4% of the total summer observation time, compared to only 14.1% in winter. In summer, the otters were seen in water at 33.7% of the sampling times, in the deep or shallow pool or in the river in the enclosure, compared to 15% in winter. In both seasons, the time budget also included 32-34% active behaviours on land, 15-17% maintenance, 5-8% affiliative social interaction and 2-3% being out of sight. In winter, the otters were more aggressive (winter 2% > summer 1%) and less active, with significantly more time spent lying down resting or sleeping (winter 11% > summer 4.6%) or being vigilant, looking around or ‘begging’ at the keeper or visitors (winter 12.2% > summer 5.8%). Feeding anticipatory activity was seen in both seasons. Affiliative social interaction occurred mainly between feeds, linked to rest periods. The relevance of these observations is discussed in relation to thermoregulation and possible effects on reproduction. |
| Keywords: swimming; feeding anticipation; temperature; thermoregulation. |
| Française | Español |
INTRODUCTION
Asian small-clawed otters (Aonyx cinereus) are small semi-aquatic animals, with distinctive hand-like front paws with reduced claws, well adapted for catching and handling small prey and other objects (Larivière, 2003). In the wild, they have a large distribution range in the tropics, from southern and south-eastern mainland Asia, through to Indonesia, Taiwan and Philippines, in diverse habitats, including coastal and freshwater wetlands, and rivers and lakes in forested areas (Wright et al., 2015). The species has been listed on CITES Appendix II since 1977, has ‘vulnerable’ status on the IUCN Red List since 2008 and is at risk of becoming regionally extinct in some areas, due to rapid population decline and loss of genetic variation, caused mainly by habitat destruction, water pollution, decline in suitable prey and direct exploitation by humans (Wright et al., 2015). In situ conservation actions include establishing networks of Protected Areas and Protected Species status for Aonyx cinereus in many range countries (Wright et al., 2015).
Zoos, aquaria and wildlife parks around the world also play an important role in species conservation and public education (Moss et al., 2015). Asian small-clawed otters (ASCO) are attractive displays due to their playful and curious nature (Foster-Turley and Markowitz, 1982; Anderson et al., 2003) and are the most common captive otter species in the United Kingdom (Wright, 2003). They cope well with different regional climatic patterns, in both indoor and outdoor enclosures, provided that they have dry shelter and a source of radiant heat in the cold season (AZA, 2009). Temperatures of 22.2-24.4°C for air and 18.3-29.4°C for water are recommended, as well as diverse water and land enclosure features, to provide adequate complexity and stimulate naturalistic behaviours (Lombardi and O'Connor, 1998; Heap et al., 2008). Successful breeding occurs in many zoos (Sivasothi, 1998; Kruuk, 2006), while in other zoos no offspring is ever produced (Foster-Turley, 1990). Mating occurs mostly in shallow water and observations suggested that otters spend more time swimming in warmer water (AZA, 2009). In 2007, there were 86 European zoos that housed a total of 434 ASCO, mostly in outdoor enclosures (62.7%), all with water features, but water temperatures were not specified (Dornbusch and Greven, 2009). In an earlier survey, most North American zoos reported having climate controlled environments for ASCO, with ambient temperatures of 21-23 ° C, while some institutions reported heating only the pool water for ASCO to 21.1 ° C because they felt this stimulated more swimming (Reed-Smith and Polechla, 2002).
While widely acknowledged that ambient temperature is an issue for tropical species, there is little quantitative information available about the influence of seasonality on the behaviour of captive tropical otters and other mammals (Reed-Smith and Polechla, 2002; Hosey et al., 2013). The metabolic costs for thermoregulation of ASCO (body temperature 37-38°C) are higher than expected compared to other Mustelids and semi-aquatic mammals, especially in water below 16°C (Borgwardt and Culik, 1999). Studies on other Mustelids showed both positive and negative correlations between water temperature and swimming activity. Lutra lutra at a zoo in Germany used the unheated pool mainly during the summer months (Längle and Jorga, 2003), while captive and free-ranging Lutra lutra (Kruuk et al., 1994a; Kruuk et al., 1997) and free-ranging mink Neovison vison (Hays et al., 2007 as Mustela vison) living in Scotland performed longer dives and were more active in colder water. Farmed mink swam more frequently in an outdoor pool during the warmer months of the year in Denmark (Hansen and Jeppesen, 2001), but warmer water up to 32°C in indoor conditions did not induce more swimming (Hansen and Jeppesen, 2003).
This study provides quantitative information for the changes from summer to winter in the behavioural time budgets, enclosure use and diurnal activity pattern of the ASCO at the Blue Reef Aquarium, Tynemouth (BRAT), England (55° N latitude; temperate climate), over a period of time when no changes in husbandry practices were implemented. The main hypothesis tested was that the otters would spend more time in the water and be more active during the warm season, due to lower overall thermoregulatory costs. The work was part of a longer-term project aiming to establish behavioural indicators of welfare for different species at this Aquarium (Cuculescu-Santana et al., 2014).
MATERIALS AND METHODS
Otters, Enclosure and Enrichment
The two Asian small-clawed otters studied were 7-8 years old male siblings (captive born in autumn 2005), around 3 kg weight, housed in an indoor enclosure of approximately 100m2 surface area, 3:2 land to water ratio, with concrete ground. Structural enrichments included a river, shallow pool and large pool (0.8 m depth; 18,000 l volume; filtered recirculated water), climbing structures, log bridges, wooden blocks with a heat lamp above them and a den (Figure 1). The otters were fed three times a day at 9am, 1pm and 4pm, on a diet of fish, day-old chicks and red meat, plus smaller random scatter feeds (monkey nuts, chopped carrot and apples, crustacean claws, molluscs) (approximately 600g/ otter/ day in total; same schedule and range of food items all year round; slightly larger random scatter feeds in the cold season). The feeds were delivered on time, as recommended by AZA (2009), to minimize stress. Feeding enrichment strategies included varying the form of delivery of the main feeds (scattered or hidden in different areas of the enclosure, to encourage foraging, or keeper-fed during training) and the type of food. A training session and public talk took place daily, combined with the 1pm feed and occasionally with the 4pm feed (Blue Reef Aquarium, 2013).
 |
| Figure 1. The otter enclosure at the Blue Reef Aquarium Tynemouth, UK. (click for larger version) |
The enclosure area was not climatise-controlled, apart from the heat lamp above the otters' preferred sleeping area, and the air and water temperatures varied in correlation with the outdoor fluctuations. In 2013, the average outdoor temperatures for Tynemouth (55° N) were 16-17°C for July-August (above the historic averages of 14-14.5°C) and 6-7°C for November-December (historic averages of 4-6°C) (ClimatEvo, 2016). The otter pool water temperatures (recorded daily) varied between 14.8-20°C in summer and 4.6-16.4°C in winter months. Natural light entered only through a small window. Artificial light was provided 8:00am-6:00pm (10 hours light:14 hours dark; metal halide bulbs with UVB qualities).
Data Collection
Data were collected by the same observer on four random days in July-August 2013 (water temperature 19.1±0.1°C ) and four random days in November-December 2013 (water temperature 11.4±1.1°C ), at nine different times each day, for 20 minutes at a time (described as ‘summer' and ‘winter' data, respectively), using instantaneous time sampling: focal for state behaviours (Table 1) and scan for location in the enclosure (Table 2) (Martin and Bateson, 2007). At the start of each observation period the otter nearest to the observer was chosen as the focal animal and its behaviour recorded every 15 seconds. The location of each otter was recorded every 60 seconds (two locations/ sampling time). The event behaviours (Table 1) were recorded using continuous observation and the one-zero rule for each 1-minute interval of observation time (Rees, 2015). The approximate visitor numbers were recorded for each 1-minute interval using a ranked score from 1 to 5 (1=a few; 2=several; 3=many; 4=full room; 5=crowded room). The methods of data collection and the ethogram (Table 1) were developed through shorter-term projects (Bowe, 2013; Briggs, 2013; Geraughty, 2014).
|
|
|
| Table 1: Otter ethogram. State behaviours and event behaviours (adapted from Hawke et al ., 2000 and Williams et al. , 2012). | |
|
|
|
| State Behaviours | Description of Behaviour |
| Swimming | Locomotion in water, with the head in or out of water, including looking for food in the water; |
| Running | Faster locomotion on land (with head up); |
| Walking/Climbing | Slower locomotion on land, on flat areas or climbing on higher structures (with head up); |
| Foraging | Moving on land with the head down and the nose close to the ground, interpreted as searching for food; |
| Scent Marking | Rubbing a body part against the ground, a structure or a wall, including sprainting; |
| Aggression | Rough fighting or other aggressive displays towards another otter; |
| Maintenance | Eating, drinking and self-grooming (using paws or mouth to clean, dry or smooth fur; gentle scratching); |
| Playing | Non-aggressive playful interaction with another otter, including play fighting, or with an object other than food, e.g. pebble, plastic toy; |
| Vigilance | Being alert, looking around while stationary either sitting or standing on hind limbs; |
| Body Rubbing | Rubbing own body against the body of another otter; non-aggressive body contact; including sexual behaviour; |
| Social Grooming | Grooming another otter (using paws or mouth to clean, dry or smooth fur); |
| Resting & Sleeping | Lying down with head down, eyes open or closed; occasionally looking around when a noise occurs; |
| Out of Sight | Hidden from the view of the observer |
| Event Behaviours | Description of Behaviour |
| Begging | Standing on hind limbs, forepaws held in front of body; repetitive up and down movements; |
| Short Calls | Short and fairly sharp repetitive sounds; |
| Long Squeals | Longer, higher pitched sounds or long mewling sounds; |
| Keeper Interaction | Direct contact with the keeper; responding to instructions from the keeper during training; |
| Yawning | Opening the mouth wide to take in air (presumed involuntary action); |
|
|
|
|
|
|||
| Table 2: Areas of the otter enclosure and their estimated biological relevance, based on their similarity to natural habitat features and compliance to the husbandry guidelines for the species (AZA, 2009). | |||
|
|
|||
| Location | Description | Biological Relevance | |
| Ground Area | Pebble-like concrete surface around the enclosure (no areas with soft substrate for digging in 2013) | Neutral | |
| Climbing Structures | Long tree branches, in a pyramid over the small shallow pool, platform at the top; thicker logs linking the ground area to the log bridges and to a large bridge over the river; wall around small pool; rocks | High | |
| Log Bridges | Three horizontal thin logs, supported by artificial tree stumps immersed in the large pool, forming a ‘path' over the large pool | High | |
| Sleeping Places | Wooden blocks stacked under a heat lamp, backed against a wall; wire mesh open cage | Moderate | |
| Den | A small house providing shelter for the otters out of sight of the visitors, with straw bedding inside | High | |
| River | Shallow flowing water with waterfall to the small pool | High | |
| Small Pool | Shallow round pool, with waterfalls to the large pool | High | |
| Large Pool | Deeper curved shoreline pool, with clear side for underwater viewing | High | |
|
|
|||
DATA PROCESSING AND DATA ANALYSIS
State behaviour data were converted into times spent engaged in each behaviour, during each observation period, and used to produce average behavioural budgets (n=36) and day-time patterns of activity (n=4) for each season (presented as % of observation time). The event behaviour data were processed as frequency of 1-minute intervals during which they occurred per observation period (presented as % of total number of 1-minute intervals per season or per time of day/season). The frequency and length of swimming and resting bouts were estimated by counting the total number of 1-minute intervals during which these behaviours were recorded 4, 3, 2, 1 or 0 times, for each season. Location data were processed as frequency of occurrence at each location per observation period (presented as % of total number of locations recorded per season or per time of day/season). The data were also combined to calculate the total number of sampling times at which the otters were in or out of water, and together or in different places (shown as % of total sampling times). Visitor data were processed as average scores per observation period, time of day and season.
The Mann-Whitney U test for the influence of season and Kruskal-Wallis test for the influence of time of day on otter behaviour were carried out in SPSS V22, at level of significance P <0.05, using the actual time and frequency values. The influence of season on location frequency data was analysed using the two-way chi-square test in Microsoft Excel 2010, at P <0.05 level of significance.
Ethical Considerations
The project received ethical approval from the Ethics Commission of Northumbria University and was carried out with consent from and in collaboration with the Displays Manager and the Keepers at the Blue Reef Aquarium Tynemouth. The data were collected from outside the enclosure, without any direct interaction between observer and otters.
RESULTS
Seasonal Differences in Otter Behaviour and Enclosure Use
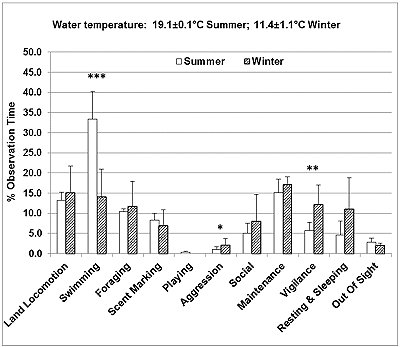
The seasonal time budgets of the otters studied are presented in Figure 2. The otters spent significantly more time swimming (33.4%) in summer, compared to winter (14.1%) (Z=-3.892, P <0.001). In contrast, in summer the otters spent less time resting and sleeping (4.6%) compared to winter (11%), and significantly less time engaged in aggressive behaviour (Z=-1.967, P <0.05) and vigilance (Z=-2.837, P <0.01). Other behaviours that increased from summer to winter were land locomotion (running, walking and climbing), foraging, affiliative social interactions (body rubbing and social grooming) and maintenance behaviours. Scent marking and being out of sight decreased slightly from summer to winter. Playing with objects was seen only in summer.
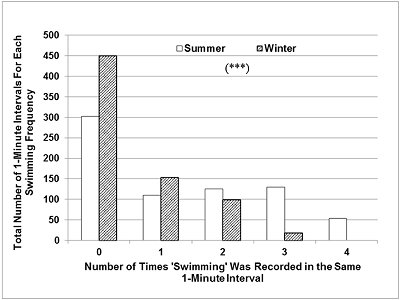
While awake and active, the otters changed behaviour frequently. Swimming usually occurred in short bouts, of 10-30 s, interspersed with 5-30 s of other active behaviours on land (e.g. walking, running or eating). The total number of 1-min intervals during which swimming was recorded 3 or 4 times (focal instantaneous time sampling every 15 s) was significantly higher than expected in summer (Figure 3), while in winter there were more than expected 1-min intervals with no swimming or swimming recorded only once (Chi-square=176.9, P <0.001, d.f.=4).
The number and length of the ‘blocks' of two or more consecutive 1-min intervals during which swimming was recorded 3 or 4 times were much greater in summer than in winter (Table 3). The opposite was seen for periods of inactivity. When the otters settled down to rest and sleep, the passive state was maintained for around 20-30 minutes, occasionally interrupted by short bouts (5-30s) of grooming, body rubbing, or looking around. The periods of continuous rest were slightly more frequent and much longer in winter (Table 3).
The otters were seen ‘begging' significantly less frequently in summer (Figure 4), during 3.8% of the total number of 1-minute intervals, compared to 20% of all 1-minute intervals in winter (Z=-2.255, p<0.05). Short calls also occurred less frequently in summer, during 38.1% of all 1-minute intervals, compared to 47.8% in winter.
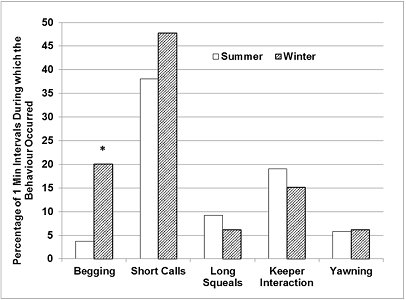 |
| Figure 4. Seasonal differences in the relative frequencies of event behaviours (720 x 1-Minute intervals/ season). Asterisks denote statistical significance at P =0.05*. (click for larger version) |
The seasonal differences in enclosure use (Figure 5) correlated well with the main differences in behaviour described above. In summer, the otters were seen in the water, in the large pool, small pool or river at 33.7% of the sampling times, which was more than double the value for winter (15%), and significantly more frequently than expected, while the climbing structures, log bridges and the sleeping places were used less frequently than expected (Chi-square=199.2, P <0.001, d.f.=7).
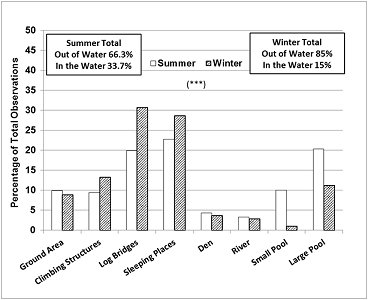 |
| Figure 5. Seasonal differences in enclosure use (720 observations/ season/ otter = 1440 observations/ season). Asterisks denote statistical significance at P =0.001***. (click for larger version) |
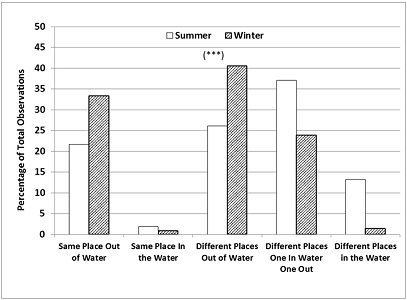
The season also had a significant influence on the frequency of occurrence of all the possible combinations of relative locations of otters to each other, in or out of water (Chi-square=132.9; p<0.001, d.f.=4). In summer, they were seen either both in the water (same or different places) or one in water and one out more frequently than expected. The otters were seen in different places at the majority of the sampling times (Figure 6) in both seasons (76.4% in summer > 65.8% in winter).
Day-Time Activity and Enclosure Use Patterns
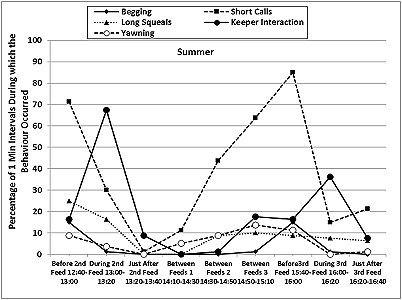
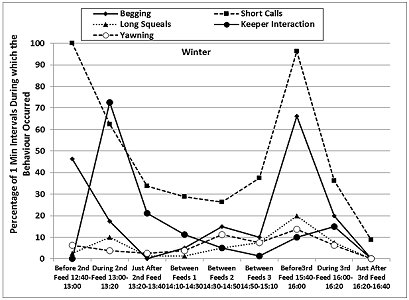
The otters displayed a fairly consistent activity pattern on the days when data collection was carried out, with peaks in locomotion (running, walking climbing, swimming) and vigilance prior to the feeding times in both seasons, as well as peaks in the frequencies of ‘begging', short calls and long squeals (Table 4, Figures 7 and 8). The peaks in vigilance and in the frequencies of short calls and ‘begging' were higher in winter than in summer for the majority of the time intervals monitored.
During feeding times, the main activities were eating, drinking and self-grooming (maintenance), foraging around the enclosure, and interaction with the keepers, with a decrease in the frequency of the short calls and the ‘begging' displays. Foraging, drinking and self-grooming continued into the periods of time described as ‘Just After Feed', with more foraging in winter (25.9% winter > 10.9% summer after 2nd feed). The maintenance activities recorded during the ‘Between Feeds' periods in both seasons consisted mainly of self-grooming, carried out while the otters were settling down to sleep or had just woken up. Occasionally, scattered food was consumed between the main feeds.
The otters were in different places almost all the time before and during the feeds, in both summer and winter. They were in the same place for most of the time during the ‘Between Feeds 1' periods in both seasons (68.8% in summer; 61.3% in winter) (Table 5). In summer they were also seen in the same place for most of the ‘Just After 2nd Feed' period (67.5% summer > 41.3% winter), when they usually settled down to rest sooner after the feed than in winter. In winter, the otters were seen in the same place more frequently than in summer, correlated with longer periods of rest and social interaction between the feeds and sooner after the 3rd feed than in summer.
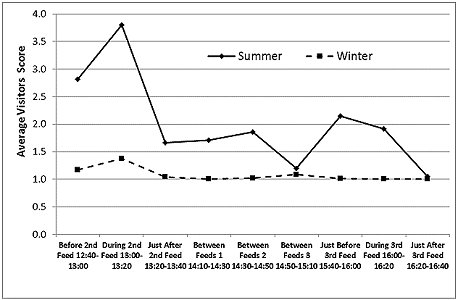
Visitor Numbers
Visitor numbers, recorded as a score (Figure 9), were higher around feeding times, especially around the second feed at 1:00pm, when otter training and a public talk were also delivered. In summer, the visitor numbers were higher than in winter at all times of day, with the exception of the interval around 3:00pm, the time of the seal and sea-lion show, when they were low in both seasons.
DISCUSSION
The indoor otter enclosure at the Blue Reef Aquarium Tynemouth (BRAT) provides various land and water features, in good compliance with the care recommendations for Asian small-clawed otters (ASCO) (AZA, 2009). Both the air and water temperatures were subject to seasonal variations, influenced by the outdoor air temperatures.
The otters studied performed mainly active behaviours and used well the space in the enclosure. As expected, they spent significantly more time swimming in summer compared to winter (33.4% > 14.1%). In winter they spent more time resting or sleeping (11% > 4.6%), displaying vigilance (12.2% > 5.8%) and being aggressive (2% > 1%). These findings were consistent with the analysis of the enclosure use. While in summer the otters were seen more frequently in the water (33.7% > 15%), in winter they were seen more frequently at the sleeping places (28.7% > 22.8%) and on the climbing structures and log bridges preferred for land locomotion and vigilance (52.7% > 39.2%). The sleeping places were also used for grooming, body rubbing and scent marking, which took place prior to settling down for resting and sleeping close to each other, usually in direct body contact, as reported by other authors for captive (Zgrabczynska and Ziomek, 2002) and wild otters (Reed-Smith et al., 2014).
In addition, our location data provided quantitative support for the keepers' observations that one of the otters spent more time swimming than the other. The combination ‘different places, one in water one out' was the most frequent occurrence in summer (37.1% of sampling times) and relatively frequent in winter (23.9%).
Overall, the active behaviours represented 87-92.4% of our total observation time. Apart from swimming, the otters displayed 32-34% active behaviours on land, 15-17% maintenance (eating, drinking and self-grooming), and 5-8% affiliative social interaction. They were out of sight for only 2-3% of the time.
In November-December 2012, the same two otters spent 10.2-13.1% of the observation time swimming, with a total of 68.8-81% active behaviours (Bowe, 2013; Briggs, 2013), similar to the winter 2013 data presented in this article. Most other studies of captive ASCO behaviour focused on the effects of feeding enrichment on foraging and stereotypic behaviour and reported lower proportions of time spent engaged in active behaviours, of 21-35%, including 8% swimming (Hawke et al., 2000; outdoor enclosure at Adelaide Zoo, 34.9 ° S), 27-41% (Ross, 2002; indoor enclosure at Lincoln Park Zoo, Chicago, 41.8 ° N) and 64-69% (Gothard, 2007; outdoor enclosure), depending on the phase of their study. The remainder of the time budgets presented by these authors comprised of inactivity or being out of sight. The periods of data collection in these studies were longer, from early morning until late afternoon, while our observations at the BRAT were carried out from midday until late afternoon, when the otters were more active. The closest value to our data was that reported for ASCO in an outdoor enclosure at Poznan Zoo, Poland (52.4 ° N), of approximately 80% active behaviours during observations before and after the single feed of the day (Zgrabczynska and Ziomek, 2002). ASCO in an outdoor enclosure at Chester Zoo, England (53.2 ° N) displayed 63-64% active behaviours, with swimming representing only 3-4% of the otters' time budget (Owen, 2004), around three times lower than our winter values for the indoor otters at the BRAT.
Data on enclosure use by ASCO are not available from other authors. The otters in this study spent the majority of the time (62-67%) in areas of the enclosure described as biologically relevant, 23-29% at preferred sleeping places, in full view of visitors and keepers, and 9-10% in neutral areas (concrete ground). The small proportion of time spent out of sight in the den (4% of the location data) was associated with defecation. In the wild, ASCO spraints are also found predominantly in areas with forest cover and less human activity (Hussain et al., 2011).
We observed that the otters used the water features frequently in both seasons, but they rarely spent more than 10-30 seconds at a time in water in winter, while in the summer there were more periods of continuous or almost continuous swimming of 1-2 min, possibly longer. The average winter water temperature on the days when the data were collected (11.4±1.1 °C) was below the recommended range of 18.3-29.4 °C (AZA, 2009), while in summer it was just above the lower extreme of this range (19.1±0.1 °C). ASCO can cope well with air temperatures of 10-15 °C, if they have a heated indoor area, but many observations suggested they swim more in warmer water (AZA, 2009), with potential beneficial effects on their health (Petrini, 1998). A female ASCO was seen swimming for up to 8 minutes at Singapore Zoo in May (1.3 ° N; air temperatures 26-30 ° C) (Nair and Agoramoorthy, 2002). The AZA Otter Care Manual states that some otters, but not all, were reported by their keepers to have reduced activity or increased food consumption during the cold season (AZA, 2009), to compensate for the higher thermoregulatory costs, but no quantitative data were given in support of this statement.
Our results provide quantitative information to support these statements and represent a potentially important observation in the context of husbandry practices that aim to foster high welfare and species conservation (Bishop et al., 2013). ASCO take well to captivity in zoos and aquaria at several locations outside the natural range of the species, in indoor and outdoor enclosures (Kruuk, 2006; Dornbusch and Greven, 2009), but reproduction is not always successful (Reed-Smith, 1998).
In the wild, ASCO live in habitats ranging from coastal wetlands to mountain streams at 2000m altitude, demonstrating high climatic and trophic adaptability (Rosli et al., 2014) and there are also reports of them living in the wild in England, after escaping from captivity (Jefferies, 1989, 1991, cited in Wright et al., 2015). Although not much is known about mating in the wild, captive ASCO are known to mate mostly in shallow water and only occasionally on land (Hussain et al., 2011) and to be able to reproduce all year round (Bateman et al., 2009). After the initiation of a Species Survival Plan ( Foster-Turley et al., 1990) and an international studbook for ASCO (Foster-Turley and Engfer, 1988), about half of the zoos with captive ASCO reported successful reproduction, with around 40 births/year and 75% cub survival (Sivasothi, 1998). An increase in the amount of energy required for thermoregulation during the cold season in zoos outside the tropical area is very likely to affect the otters' energy budget, with consequences on behaviour, nutritional needs, and possibly reproduction.
Borgwardt and Culik (1999) showed that while ASCO had similar metabolic rates to other otters on land, in water they had much higher metabolic rates than sea otters and river otters (Pfeiffer and Culik, 1998), reflecting higher thermoregulatory costs, possibly due to poorer insulative properties of the fur and underlying skin and less well-developed heat saving mechanisms in the vascularisation of their appendages (Borgwardt and Culik, 1999) or pattern of movement during swimming (Fish, 1994). The metabolic rate of ASCO with a body mass of around 3 kg was 5.0±0.8 W kg -1 during rest on land at 16 °C, almost doubled to 9.1±0.8 W kg -1 during rest in colder water at 12 °C, and increased further to 14.2±3.9 W kg -1 during swimming, and 17.6±1.4 W kg -1 during foraging and feeding in water at 12 °C (Borgwardt and Culik, 1999).
In spite of the special features of the hairs of Mustelids that can interlock and trap an effective insulating air layer around the body (Kuhn and Meyer, 2010; Liwanag et al., 2012), the heat loss from the skin increases rapidly through forced convection during movement in cold water (Hind and Gurney, 1997), and the heat generated in muscles during locomotion cannot fully compensate for the lost heat (Humphries and Careau, 2011).
The metabolic rate of the much larger Lutra lutra also increased in cold water (Kruuk et al., 1994a), but the length of time spent foraging in water (mean bout length 32.5 min) and the rate of decrease in body temperature (2.3 °C hr -1 ) were not significantly influenced by water temperatures in the range 2-16 ° C (Kruuk et al., 1997). The smaller wild mink Neovison vison were also more active and dived more in cold water (4-6 ° C), but with shorter dive durations up to 60s (Hays et al., 2007; Harrington et al., 2012).
Kruuk et al. (1997) showed that Lutra lutra increased their body temperature through more activity on land before a foraging dive, then exited the water when their body temperature decreased again to a critical value, equivalent to their average resting value. A similar regulatory mechanism in ASCO may explain the less frequent shorter swimming bouts we observed in water at 11-12 ° C (winter), compared to water at 19 ° C (summer) and may also contribute to the poor reproductive success of this species in captivity (Foster-Turley, 1990). Copulation is known to last 5-30 minutes in shallow water (Heap et al., 2008). No management practices were reported to significantly increase the chance of successful breeding (Reed-Smith, 1998), but rapid heat loss in cold water may determine some ASCO to exit water before successful insemination occurs. The faster rate of heat loss of ASCO may represent an evolutionary adaptation to the tropical range (Kruuk, 2006), where overheating during activity may be a more frequent occurrence than unfavourable heat loss. Thermal imaging showed that another tropical species, the giant otter Pteronura brasiliensis , lost heat through the entire body surface, including the tail, while the Eurasian otters Lutra lutra dissipated excess heat only through their feet (Kuhn and Meyer, 2009).
The patterns of activity and enclosure use presented in this study appear to support this idea. The greatest difference between summer and winter was recorded for the observation periods after 2:00pm, when swimming represented 2-7 times more of the time budget (28.8-53.8%) than during the same time periods in winter (Table 4). These were also the periods when the longer bouts of swimming occurred in the summer, when the otters were seen more frequently in the water at the same time (8.8%-37.5%), usually one swimming in the large pool and the other sitting in the shallow pool, and when the indoor air and water temperatures were warmer by 1-2 ° C and 0.5-1 ° C, respectively, compared to the morning values. This suggested that, in spite of different tendencies to swim, both otters were using the water for thermoregulation in the summer, to keep cool or to cool down after land locomotion. In winter, the otters were seen in the water significantly less frequently, and they were rarely both in the water at the same time (0-5% of sampling times).
Our results also showed that while active, the two male otters usually kept their distance, both on land and in water, in both seasons, suggesting a tendency for territoriality or dominance. This was associated with frequent scent marking after the enclosure was cleaned. Scent marking plays an important role in the communication of identity and social and reproductive status between otters and in establishing and maintaining territories, with several reports of territorial and mutual avoidance (Hussain et al., 2011; Rostain et al., 2012). Recent studies demonstrated that the acoustic and olfactory identities of male ASCO were more important for their recognition by females than their visual appearance (Lemasson et al., 2013), although ASCO have the ability to discriminate color cues (Svoke et al., 2014). A group of all female ASCO at Chester Zoo, England, displayed very little scent marking, much less than a mixed sex family group at the same zoo (Foster and Fletcher, 2007).
Individual differences in tendency to swim were documented in both captive and free-ranging otters (Kruuk et al., 1994a; Kruuk et al., 1997) and mink (Hansen and Jeppesen, 2001; Hays et al., 2007; Vinke et al., 2008; Harrington et al., 2012). In the wild, swimming is mainly associated with looking for food and ASCO, although otherwise highly social and gregarious, were often seen foraging on their own (Kruuk, 2006). Captive mink also appeared to choose to swim for increased foraging and behavioural choices, rather than thermoregulation (Vinke et al., 2008).
This could be related to our observations that swimming was also part of the feeding anticipatory activity (FAA) of ASCO at the BRAT, in both seasons. FAA started at least 20 minutes before the scheduled feeding times and included running, walking, swimming, frequent ‘begging' displays and short calls and occasional long squeals and aggressive displays. Although the door banging and hair plucking described by Ross (2002) were not seen, the otters' movements followed fairly repetitive trajectories, and could be considered stereotypic behaviour (Gothard, 2007; Morabito and Bashaw, 2012). One otter spent more time swimming in the large pool, while the other spent more time running and ‘begging' on the log bridges.
Similar patterns of FAA were described for captive ASCO (Hawke et al., 2000; Ross, 2002), and for farmed mink (Hansen and Møller, 2008; Axelsson et al., 2009). In captive carnivores, feeding anticipatory activity is entrained primarily by the regularity of feeding times (Mistlberger, 2011). Several studies showed that fixed feeding times align the circadian clock gene rhythms in peripheral organs and in many areas of the brain with the daily rhythm of food intake (Carneiro and Araujo, 2012).
The peaks in locomotory activity prior to feeding at the BRAT were interpreted as appetitive behaviour associated with good welfare, as proposed by Watters (2014), and they also attracted and retained the visitors' interest. More visitors stopped at the otter enclosure just before and during feeds in both seasons. Other studies also showed that visitors spent longer times at the otter enclosures when the animals were more active and a public talk was taking place (Anderson et al., 2003; Bowe, 2013; Briggs, 2013). Circadian patterns of activity and vocalisation exist in wild otters and are most likely synchronised with prey availability, as well as climatic conditions and degree of human interference (Hussain, 2013). ASCO were heard ‘chirping' during the night and were occasionally seen early in the morning and around dusk (Foster-Turley, 1992; Castro and Dolorosa, 2006). The larger smooth-coated otters Lutrogale perspicillata , a closely related (Rosli et al., 2015) sympatric species (Kruuk et al., 1994b), had two periods of high activity during the night, separated by a longer period of relative inactivity during the day in the summer (daytime temperatures up to 46 ° C) and were more active and more diurnal during winter, with three peaks of activity (Hussain, 2013). The spotted necked otter Lutra maculicollis (Lichtenstein, 1835), a diurnal African otter of comparable weight to ASCO, had activity peaks during morning (33% of time budget) and late afternoon (23% of time budget) (Reed Smith et al., 2014). The main activities seen were travelling, foraging and grooming (Hussain, 2013; Reed-Smith et al., 2014) or playing, running and swimming (Castro and Dolorosa, 2006).
The higher peaks in ‘begging' and calling prior to feeding times observed at the BRAT in winter, combined with the extended foraging after the 1pm feed, suggested that these were more likely to be signs of hunger, as proposed by Gothard (2007), and that introduction of another feed or more substantial scatter feeds in the cold season would be beneficial. Standing on hind limbs to look around was observed in wild ASCO and smooth coated otters, and was associated with a state of alarm and vigilance to threats (Kruuk, 2006). Gothard (2007) and Owen (2004) showed that ASCO “begged” and called more in the presence of visitors, which is similar to our observations for each season. However, in our study, the visitor numbers were much higher in summer, during the school holidays, when the overall frequency of ‘begging' was significantly lower than in winter. Otters have a complex vocal repertoire that can convey biologically important messages (Lemasson et al., 2014) and pre-feeding short calls were categorised as signs of stress in captive ASCO (Scheifele et al., 2015), consistent with the fact that otters have high metabolic rates and fast food transit and become hungry again soon after being fed (Hawke et al., 2000). If the higher nutritional requirements associated with higher thermoregulatory costs during the cold season (Henry et al., 2012) are not fully met, this is likely to lead to increases in stereotypic and passive behaviour from summer to autumn-winter, as those described in this article and those reported by Ahola et al. (2011) for captive mink. In addition, play behaviour that has been proposed as an indicator of favourable energy balance and high otter welfare (Gothard, 2007), was seen at the BRAT only in the summer.
CONCLUSION
- This is the first quantitative study demonstrating a significant influence of seasonality on the behaviour and enclosure use of Aonyx cinereus in captivity at a location outside the species natural range;
- The otters at the BRAT spent significantly more time swimming and significantly less time displaying stereotypical behaviours during the warm season, compared to the cold season, suggesting that captive Aonyx cinereus kept in non-climatised indoor enclosures in zoos and aquaria in temperate regions may benefit from having at least partially heated pools during the cold season, to stimulate activity all year round and increase visitor display value;
- Access to warmer water in such enclosures may also have a positive influence on their reproductive success in zoos in temperate climate regions;
- Further studies on a larger number of ASCO at different establishments, including some with outdoor enclosures, and for longer periods of time would be required to reach stronger conclusions regarding these aspects of captive behaviour.
Acknowledgements: The authors would like to thank Joseph Ioanna, Emma Quinn, Steph Taylor and all staff at the Blue Reef Aquarium Tynemouth for their enthusiasm and support with this project. The work was carried out within the standard workloads of staff and students at Northumbria University and staff at the Blue Reef Aquarium Tynemouth.
REFERENCES
Ahola, L., Mononen, J., Mohaibes, M. (2011). Effects of access to extra cage constructions including a swimming opportunity on the development of stereotypic behaviour in singly housed juvenile farmed mink ( Neovison vison ). Appl. Anim. Behav. Sci. 134: 201-208.
Anderson, U.S., Kelling, A.S., Pressley-Keough, R., Bloomsmith, M.A., Maple, T.L. (2003). Enhancing the zoo visitor's experience by public animal training and oral interpretation at an otter exhibit. Environ. Behav. 35: 826-841.
Axelsson, H.M.K., Alden, E., Lidfors, L. (2009). Behaviour in female mink housed in enriched standard cages during winter. Appl. Anim. Behav. Sci. 121: 222-229.
AZA (2009) . Otter (Lutrinae) care manual . Silver Spring, MD: Association of Zoos and Aquariums. 154 p.
Bateman, H.L., Bond, J.B., Campbell, M., Barrie, M., Riggs, G., Snyder, B., Swanson, W.F. (2009). Characterization of basal seminal traits and reproductive endocrine profiles in North American river otters and Asian small-clawed otters. Zoo Biol. 28: 107-126.
Bishop, J., Hosey, G., Plowman, A. (2013) . Handbook of zoo research. Guidelines for conducting research in zoos. London. BIAZA. 231 p.
Blue Reef Aquarium (2013) . Daily schedule. Downloaded on June 20, 2013. Available from http://www.bluereefaquarium.co.uk/tynemouth/ .
Borgwardt, N., Culik, B.M. (1999) . Asian small-clawed otters ( Amblonyx cinerea ): resting and swimming metabolic rates. J. Comp. Physiol. B 169: 100-106.
Bowe, C. (2013) . Behavioural study of the Asian short-clawed otter (Aonyx cinerea) in captivity at the Blue Reef Aquarium Tynemouth . [B.Sc. Dissertation]. Newcastle upon Tyne: Northumbria University. 57 p.
Briggs, R.N. (2013). A study exploring the behaviour of the Asian short-clawed otters ( Aonyx cinerea ) and the enrichment strategies used for this species at the Blue Reef Aquarium Tynemouth . [B.Sc. Dissertation]. Newcastle upon Tyne: Northumbria University. 89 p.
Carneiro, B.T.S., Araujo, J.F. (2012). Food entrainment: Major and recent findings. Front. Behav. Neurosci. 6: 83. doi: 10.3389/fnbeh.2012.00083.
Castro, L.S.G., Dolorosa, R.G. (2006) . Conservation status of the Asian small-clawed otter Amblonyx cinereus (Illiger, 1815) in Palawan, Philippines. Philipp. Sci. 43: 69-76.
ClimatEvo (2016). Weather statistics and climate changes. Downloaded January 9, 2016. Available from http://climatevo.com/ .
Cuculescu-Santana, M., Bowe, C., Briggs, R.N., Chorlton, C., Fitzpatrick, A., Geraughty, M., Haigh, S., Minvalla, A., Woolley, G. and Horn, C. (2014). The effect of enrichment on the behaviour of otters, monkeys, degus, seals and fish at the Blue Reef Aquarium Tynemouth. Northumbria University Research Conference Abstracts 21&22 May 2014 . 137 p.
Dornbusch, T., Greven, H. (2009) . Notes on keeping of Asian small-clawed otters ( Amblonyx cinereus ) in European zoos. Zool. Garten 78: 75-80. [In German with English abstract].
Fish, F.E. (1994). Association of propulsive swimming mode with behaviour in river otters ( Lutra canadensis ). J. Mammal. 75: 989-997.
Foster, C.E., Fletcher, A.W. (2007). Demographic influences on the social behaviour of the female Asian short clawed otter Amblonyx cinerea in captivity. 8 th Annual Symposium on Zoo Research. BIAZA . p103-104.
Foster-Turley, P. (1990). Otters in captivity. In: Foster-Turley, P., Macdonald, S., Mason, C. (eds.) Otters. An action plan for their conservation . Proceedings of the IUCN Otter Specialist Group Meeting, Gland, Switzerland. p 17-21.
Foster-Turley, P. (1992). Conservation aspects of the ecology of Asian small-clawed and smooth otters on the Malay Peninsula. IUCN Otter Spec. Group Bull. 7: 26-29.
Foster-Turley, P., Engfer, S. (1988). The species survival plan for the Asian small-clawed otter ( Aonyx cinerea ). Int. Zoo Yearbook 27: 79-84.
Foster-Turley, P., Macdonald, S., Mason, C. (1990). Otters. An action plan for their conservation . Proceedings of the IUCN Otter Specialist Group Meeting, Gland, Switzerland. 133 p.
Foster-Turley, P., Markowitz, H. (1982). A captive behavioural enrichment study with Asian small-clawed river otters ( Aonyx cinerea ). Zoo Biol. 1: 29-43.
Geraughty, M.L. (2014). A study investigating the effect of environmental enrichment on the behaviour of the Asian Small-Clawed Otters at the Blue Reef Aquarium Tynemouth . [B.Sc. Dissertation]. Newcastle upon Tyne: Northumbria University. 62 p.
Gothard, N. (2007). What is the proximate cause of begging behaviour in a group of captive Asian short-clawed otters? IUCN Otter Spec. Group Bull. 24: 14-35.
Hansen, C.P.B., Jeppesen, L.L. (2001). Use of water for swimming and its relationship to temperature and other factors in farm mink ( Mustela vison ). Acta Agr. Scand. A-An. 51: 89-93.
Hansen, C.P.B., Jeppesen, L.L. (2003). The influence of temperature on the activity and water use of farmed mink ( Mustela vison ). Anim. Sci. 76: 111-118.
Hansen, S.W., Møller, S.H. (2008). Diurnal activity patterns of farm mink ( Mustela vison ) subjected to different feeding routines. Appl. Anim. Behav. Sci. 111: 146-157.
Harrington, L.A., Hays, G.C., Fasola, L., Harrington, A.L., Righton, D., Macdonald, D.W. (2012). Dive performance in a small-bodied, semi-aquatic mammal in the wild. J. Mammal. 93: 198-210.
Hawke, L., Lauer, P., Bartholomeusz, D., Steen, Z. (2000). Effects of increased food dispersal and random feeding time/ place on stereotyped behaviours in otters at Adelaide Zoo. Int. Zoo News 47: 71-81.
Hays, G.C., Forman, D.W., Harrington, L.A., Harrington, A.L., Macdonald, D.W., Righton, D. (2007). Recording the free-living behaviour of small-bodied, shallow-diving animals with data loggers. J. Anim. Ecol. 76: 183-190.
Heap, C.J., Wright, L., Andrews, L. (2008). Summary of husbandry guidelines for Asian small-clawed otters in captivity . IUCN/ SSC Otter Specialist Group, Otters in Captivity Task Force. 17 p.
Henry, B., Maslanka, M., Heuer, K., Reed-Smith, J., Nidasio, G. (2012). Summary of nutrition guidelines for otters in zoos, aquaria, rehabilitation and wildlife sanctuaries . IUCN/ SSC Otter Specialist Group, Otters in Zoos, Aquaria, Rehabilitation and Wildlife Sanctuaries Task Force. 26 p.
Hind, A.T., Gurney, W.S.C. (1997). The metabolic cost of swimming in marine homeotherms. J. Exp. Biol. 200: 531-542.
Hosey, G., Melfi, V., Pankhurst, S. (2013). Zoo animals: Behaviour, management and welfare . 2nd Edition. Oxford University Press. 643 p.
Humphries, M.M., Careau, V. (2011). Heat for nothing or activity for free? Evidence and implications of activity-thermoregulatory heat substitution. Integr. Comp. Biol. 51: 419-431.
Hussain, S.A. (2013). Activity pattern, behavioural activity and interspecific interaction of smooth-coated otter ( Lutrogale perspicillata ) in National Chambal Sanctuary, India. IUCN Otter Spec. Group Bull. 30: 5-17.
Hussain, S.A., Gupta, S.K., de Silva, P.K. (2011). Biology and ecology of Asian small-clawed otter Aonyx cinereus (Illiger, 1815): A review. IUCN Otter Spec. Group Bull. 28: 63-75.
Jefferies, D.J. (1989). The Asian short clawed otter Amblonyx cinerea (Illiger) living wild in Britain. Otters (Earsham) 2 (3): 21-25.
Jefferies, D.J. (1991). Another record of an Asian short-clawed otter living free in Oxford with notes on its implications. J. Otter Trust 2 (5): 9-12.
Kruuk, H. (2006). Otters: ecology, behaviour and conservation . Oxford University Press. 265 p.
Kruuk, H., Balharry, E., Taylor, P.T. (1994a). Oxygen consumption of the Eurasian otter Lutra lutra in relation to water temperature. Physiol. Zool. 67: 1174-1185.
Kruuk, H., Kanchanasaka, B., O'Sullivan, S., Wanghongsa, S. (1994b). Niche separation in three sympatric otters, Lutra perspicillata , L. lutra and Aonyx cinerea in Huay Kha Khaeng, Thailand. Biol. Conserv. 69: 115-120.
Kruuk, H., Taylor, P.T., Mom, G.A.T. (1997). Body temperature and foraging behaviour of the Eurasian otter ( Lutra lutra ), in relation to water temperature. J. Zool. 241: 689-697.
Kuhn, R.A., Meyer, W. (2009). Infrared thermography of the body surface in the Eurasian otter Lutra lutra and the giant otter Pteronura brasiliensis . Aquat. Biol. 6: 143-152.
Kuhn, R.A., Meyer, W. (2010). A note on the specific cuticle structure of wool hairs in otters (Lutrinae). Zool. Sci. 27: 826-829.
Längle, T., Jorga, W. (2003). Untersuchungen zur optimierung der haltungsbedingungen beim Eurasischen fischotter ( Lutra lutra ). Zool. Garten 73: 11-32. [In German, with English abstract]
Larivière, S. (2003). Amblonyx cinereus . Mamm. Species 720: 1-5.
Lemasson, A., Mikus, M.-A., Blois-Heulin, C., Lode, T. (2013). Social partner discrimination based on sounds and scents in Asian small-clawed otters ( Aonyx cinereus ). Naturwissenschaften 100: 275-279.
Lemasson, A., Mikus, M.-A., Blois-Heulin, C., Lode, T. (2014). Vocal repertoire, individual acoustic distinctiveness, and social networks in a group of captive Asian small-clawed otters ( Aonyx cinerea ). J. Mammal. 95: 128-139.
Liwanag, H.E.M., Berta, A., Costa, D.P., Abney, M., Williams, T.M. (2012). Morphological and thermal properties of mammalian insulation: The evolution of fur for aquatic living. Biol. J. Linn. Soc. 106: 926-939.
Lombardi, D., O'Connor, J. (1998). Asian small-clawed otter (Aonyx cinerea): Husbandry manual. American Zoo and Aquarium Association. 112 p.
Martin, P., Bateson, P. (2007). Measuring behaviour. An introductory guide. 3rd Edition. Cambridge University Press. 176 p.
Mistlberger, R.E. (2011). Neurobiology of food anticipatory circadian rhythms. Physiol. and Behav. 104: 535-545.
Morabito, P., Bashaw, M.J. (2013). A survey of abnormal repetitive behaviours in North American river otters housed in zoos. J. Appl. Anim. Welf. Sci. 15: 208-221.
Moss, A., Jensen, E., Gusset, M. (2015). Evaluating the contribution of zoos and aquariums to Aichi Biodiversity Target 1. Conserv. Biol. 29: 537-544.
Nair, S., Agoramoorthy, G. (2002). Mating- and birth-related behaviour in captive Asian small-clawed otters. Int. Zoo News 49 (1): No. 314. 4 p.
Owen, C. (2004). Do visitors affect the Asian short-clawed otter Aonyx cinerea in a captive environment? 6th Annual Symposium on Zoo Research. BIAZA . p 202-211.
Petrini, K.R. (1998). Health care. In: Lombardi, D., O'Connor, J. (eds). Asian small-clawed otter (Aonyx cinerea): Husbandry manual. American Zoo and Aquarium Association. p. 19-47.
Pfeiffer, P., Culik, B.M. (1998). Energy metabolism of underwater swimming in river-otters ( Lutra lutra L.). J. Comp. Physiol. B 168: 143-148.
Reed-Smith, J. (1998). Reproduction. In: Lombardi, D., O'Connor, J. (eds). Asian small-clawed otter (Aonyx cinerea): Husbandry manual . American Zoo and Aquarium Association. p 49-52.
Reed-Smith, J., Polechla, P.J.Jr. (2002). Husbandry and captive breeding of otters (Lutrinae) in North and Latin America: A synopsis of current progress. Proc. VIIth International Otter Colloquium, p 276-281.
Reed-Smith, J., Serfass, T., Samweli Kihudu, T., Mussa, M. (2014). Preliminary report on the behaviour of spotted-necked otter ( Lutra maculicollis , Lichtenstein, 1835) living in a lentic ecosystem. Zoo Biol. 33: 121-130.
Rees, P.A. (2015). Studying captive animals: A workbook of methods in behaviour, welfare and ecology . Wiley-Blackwell. 301 p.
Rosli, M.K.A., Syed-Shabthar, S.M.F., Abdul-Patah, P., Abdul-Samad, Z., Abdul, S.N., Burhanuddin, M.N., Zulkifli, N.A., Shukor, M.N., Budsabong, K., Changtragoon, S., Sekiguchi, T., Sasaki, H., Md-Zain, B.M. (2014). A new subspecies identification and population study of the Asian small-clawed otter ( Aonyx cinereus ) in Malay Peninsula and Southern Thailand based on fecal DNA method. The Scientific World J. 2014: 457350.
Rosli, M.K.A., Abdul-Patah, P., Syed-Shabthar, S.M.F., Burhanuddin, M.N., Sekiguchi, T., Sasaki, H., Shukor, M.N., Yaakop, S., Md-Zain, B.M. (2015). Phylogenetic relationships of the Malay peninsula otters ( Lutra sumatrana , Lutrogale perspicillata and Aonyx cinereus ) based on DNA sequences of mitochondrial D-loop region. J. Anim. Plant Sci. 25: 836-843.
Ross, S.R. (2002). The effect of a simple feeding enrichment strategy on the behaviour of two Asian small-clawed otters ( Aonyx cinerea ). Aquat. Mammals 28: 113-120.
Rostain, R.R., Ben-David, M., Groves, P., Randall, J.A. (2004). Why do river otters scent-mark? An experimental test of several hypotheses. Anim. Behav. 68: 703-711.
Scheifele, P.M., Johnson, M.T., Fry, M., Hamel, B., Laclede, K. (2015). Vocal classification of vocalizations of a pair of Asian Small-Clawed otters to determine stress. J. Acoust. Soc. Am. 138: EL105-EL109.
Sivasothi, N. (1998). Asian otters in captivity. Asian Otter Newsletter 5: 15-18.
Svoke, J.T., Snyder, R.J., Brink Elgart, J. (2014). Preliminary evidence for color stimuli discrimination in the Asian small-clawed otter ( Aonyx cinerea ). Learn. Behav. 42: 176-184.
Vinke, C.M., Hansen, S.W., Mononen, J., Korhonen, H., Cooper, J.J., Mohaibes, M., Bakken, M., Spruijt, B.M. (2008). To swim or not to swim: An interpretation of farmed mink's motivation for a water bath. Appl. Anim. Behav. Sci. 111: 1-27.
Waitt, C., Buchanan-Smith, H.M. (2001). What time is feeding? How delays and anticipation of feeding schedules affect stump-tailed macaque behaviour. Appl. Anim. Behav. Sci. 75: 75-85.
Watters, J.V. (2014). Searching for behavioural indicators of welfare in zoos: Uncovering anticipatory behaviour. Zoo Biol. 33: 251-256.
Williams, R.L., Porter, S.K., Hart, A.G., Goodenough, A.E. (2012). The accuracy of behavioural data collected by visitors in a zoo environment: Can visitors collect meaningful data? Int. J. Zool. 2012: 724835.
Wright, L.C. (2003). Assessing the welfare of captive Asian small-clawed otters ( Amblonyx cinereus ): Can inductive methods play a part? IUCN Otter Spec. Group Bull. 20: 35-41.
Wright, L., de Silva, P., Chan, B., Reza Lubis, I. (2015). Aonyx cinereus . The IUCN Red List of threatened species 2015. Downloaded 30 January 2016. Available from http://dx.doi.org/10.2305/IUCN.UK.20152.RLTS.T44166A21939068.en .
Zgrabczynska, E., Ziomek, J. (2002). Preliminary studies on foraging behaviour and dietary preferences in a group of Asian small-clawed otter ( Aonyx cinerea ) in the Poznan Zoo. Zool. Garten 72: 189-196.
Résumé : Changements saisonniers dans le comportement et l'enclos utilisation des loutres cendrées Aonyx cinereus en captivité
L'influence des variations saisonnières de la température sur le comportement des mammifères tropicaux maintenus dans les zoos et les aquariums dans les régions du climat tempéré est très peu étudiée. Cet article décrit les différences saisonnières dans le budget de comportement et dans l'utilisation de l'enceinte de deux loutres cendrées ( Aonyx cinereus ) détenus dans une enceinte intérieure à l'aquarium Blue Reef de Tynemouth, au nord-est de l'Angleterre (55°N). Les loutres étudiées ont passé significativement plus de temps dans l'eau en été (température de l'eau 18-19°C) qu'en hiver (température de l'eau 11-12°C). La natation représentait 33,4% du temps total d'observation en été, contre seulement 14,1% en hiver. En été, les loutres ont été observées dans l'eau à 33,7% des temps d'échantillonnage, dans une de piscines ou dans la rivière dans l'enceinte, contre 15% en hiver. Dans les deux saisons, le budget du temps comprenait aussi de 32-34% de comportements actifs sur terre, 15-17% d'entretien, 5-8% d'interaction sociale affiliée et 2-3% étaient hors de vue. En hiver, les loutres étaient plus agressives (hiver 2%> été 1%) et moins actives, avec significativement plus de temps allongé au repos (hiver 11%> été 4,6%) ou vigilants, regardant autour d'eux ou ‘mendiant' aux gardiens ou aux visiteurs (hiver 12,2%> été 5,8%). L'activité d'anticipation alimentaire a été observée dans les deux saisons. L'interaction sociale affiliative s'est produite principalement entre les temps d'alimentation, liés aux périodes de repos. La pertinence de ces observations est discutée en relation avec la thermorégulation et les possibles effets sur la reproduction.
Revenez au dessus
Resumen: Cambios estacionales en el comportamiento y encuentro utilización de nutria inerme asiatica Aonyx cinereus captivas
LLa influencia de los cambios estacionales de temperatura en el comportamiento de los mamíferos tropicales mantenidos en zoos y acuarios en regiones de clima templado es muy poco estudiada. Este artículo describe las diferencias estacionales en el presupuesto de tiempo de comportamiento y en el uso del recinto de dos nutrias inerme asiatica ( Aonyx cinereus ) mantenidas en un recinto interior en el Acuario Blue Reef de Tynemouth, en el noreste de Inglaterra (55°N). Las nutrias estudiadas pasaron significativamente más tiempo en el agua en verano (temperatura del agua 18-19°C) que en invierno (temperatura del agua 11-12°C). La natación representó el 33,4% del tiempo total de observación en verano, en comparación con sólo el 14,1% en invierno. En verano, las nutrias fueron observadas en agua en 33.7% de los tiempos de muestreo, en una de las piscinas o en el río del recinto, en comparación con el 15% en invierno. En ambas temporadas, el presupuesto de tiempo también incluyó 32-34% de conductas activas en tierra, 15-17% de mantenimiento, 5-8% de interacción social afiliada y 2-3% fuera de la vista. En invierno, las nutrias fueron más agresivas (invierno 2%> verano 1%) y menos activas, pasando significativamente más tiempo acostadas, descansando o durmiendo (invierno 11%> verano 4,6%), y siendo vigilantes, mirando alrededor o 'mendigando' al guardián o visitantes (invierno 12,2%> verano 5,8%). La actividad anticipatoria de alimentación se observó en ambas estaciones. La interacción social afiliada ocurrió principalmente entre las comidas, vinculada a los períodos de descanso. Se discute la relevancia de estas observaciones en relación con la termorregulación y los posibles efectos sobre la reproducción.
Vuelva a la tapa