IUCN/SSC Otter Specialist Group Bulletin

|
©IUCN/SCC Otter Specialist Group
Volume 33 Issue 2 (October 2016) Citation: Pimenta, NC, Barnett,
APA, Lazzarini, SM, Ribeiro, DC, Ramalheira, CS and Weber Rosas, FCW (2016). Home Alone: Records of Abandonment of Still-Dependent Giant Otter (Pteronura brasiliensis)
and Neotropical Otter (Lontra longicaudis) Individuals in Brazilian Amazon. IUCN Otter Spec. Group Bull.
33 (1): 86 - 95 Home Alone: Records of Abandonment of Still-Dependent Giant Otter (Pteronura brasiliensis) and Neotropical Otter (Lontra longicaudis) Individuals in Brazilian Amazon Natalia Camps Pimenta1, Adrian Paul Ashton Barnett1, Stella Maris Lazzarini2, Daniella Carvalho Ribeiro2, Claudiane dos Santos Ramalheira3, and Fernando Cesar Weber Rosas3
1Amazon Mammals Research Group, Department of Ecology,
National Institute of Amazonian Research, Manaus, Brazil.
e-mail: natalia.camps@gmail.com; Telephone: 55 (92) 3643-3225. e-mail
natalia.camps@gmail.com |





 |
| (Received 1st September 2016, accepted 27th November 2016) |
| Abstract: Despite the increase of Lutrinae studies, the knowledge of some behaviors remains limited, especially those that are rarely seen. This study presents three cases of cub abandonment by Pteronura brasiliensis, and one for Lontra longicaudis. The three cases of giant otter abandonment occurred in nutrient poor habitats. The neotropical otter case may be related to cub illness. The current reports, and any future reports they encourage, could be important when developing conservation plans for these species, since natural deaths may have implications for population dynamics. Such reports could also contribute to an understanding of the circumstances surrounding abandonment behavior.. |
| Keywords:behavior, cooperative breeding, endangered species, Lutrinae, parental care. |
| Française | Español |
All mammalian infants require special care to survive during the initial stages of life (Thompson et al., 2010). This scenario is no different for otters. For giant otter ( Pteronura brasiliensis ), a social species characterized by the presence of one reproductive pair and non-reproductive individuals, all adult individuals in the group are responsible for helping in territory defense and for shared care of the young (Carter and Rosas, 1997). However, as non-reproductive adult are not obligate assistants, giant otter appears to occupy an intermediate position between the cooperative and non-cooperative breeding species (Rosas et al., 2009).
Usually, but not always, young cubs remain in the den with one or more adults - breeders or non-breeders - as companions or babysitters - while the rest of the group forages and patrols the group territory (Duplaix, 1980; Carter and Rosas, 1997; Staib, 2005; Rosas et al., 2009). The role of companions is to protect the cubs against potential predators, dehydration, drowning and hypothermia, and generally help raise the offspring of the reproductive pair.
The situation is somewhat different for the neotropical otter ( Lontra longicaudis ). While giant otters are social and monogamous, the neotropical otter is most often solitary and seems to have a polygamous mating system where males, which have larger territories than females, superimpose their territories on those of several females. However, according to Larivière (1999), the male remains only one day with the female, and the parental care is carried out solely by the mother.
Investment by parents in young is often very extensive in terms of time, energy, and curtailment of overall reproductive effort (Pontier et al., 1993; Charnov and Ernest, 2006). Abandoning such expensive investments should be rare, and occur only under extreme circumstances, or occur as a result of pure accident (Trivers, 1972). Consequently, in otters, the absence of adults from dens with cubs is generally observed only when there is no imminent danger (Rosas et al . , 2009). Often such absences appear to have no negative consequences. However, occasionally newborn individuals are genuinely abandoned, and this can lead to severe consequences for the individual involved, as well as repercussions for the local population. Natural deaths may play a significant role in population dynamics when a species is endangered (Duplaix et al. , 2008), and so should be considered in future management plans (Rosas and de Mattos, 2003).
To our knowledge this is the first detailed report of offspring abandonment for either of these species. A report by Staib and Schenk (1994) mentioned that abandonment might occur, but gave no concrete details. McTurk and Spelman (2005) mentioned ‘orphaned' giant otter cubs, but did not specify how they gained this status, while the solitary cub mentioned by Lima and Marmontel (2011) had survived poaching, not abandonment. In the only other publications known to us that mentions mortality in P. brasiliensis cubs, death was the result of either cannibalism or territorial conflict, but not abandonment (Mourão and Carvalho, 2001; Leuchtenberger et al., 2015).
Abandonment of young can be an adaptive strategy when continued current investment compromises overall long-term fitness (Trivers, 1972), and has been reported for a variety of larger mammals, including those with cooperative care (e.g. hunting dogs – Malcolm and Marten, 1982; lions – Eloff, 1980; Packer and Pusey, 1983; meercats - Doolan and MacDonald, 1997; Klug and Bonsall, 2007 for general review ). Abandonment of cubs has also been reported in other otter species, either in response to low food availability (Kruuk et al., 1991), cub illness (Simpson et al., 2008), or den disturbance (Jeffries, 1987; Hauer et al., 2002). However, it has not been reported for any species of otter in the Neotropics. Here, we report four instances of abandonment of young in otters from the Neotropics; three involving the giant otter, and one the neotropical otter.
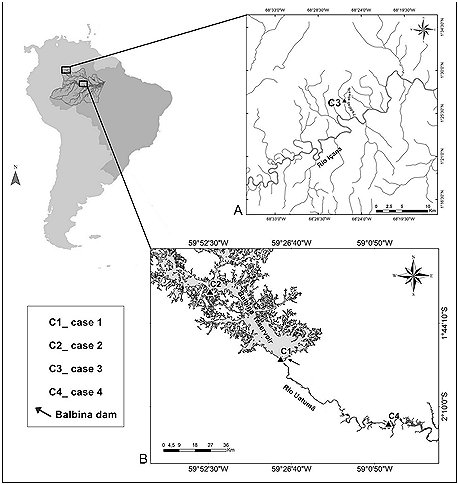
The first incident occurred in the Uatumã Biological Reserve (see Figure 1), upstream of the Balbina dam in Amazonas State, Brazil (IBAMA, 1997). On 3rd January, 1996 a solitary newborn giant otter cub was found by fishermen. The individual was on a muddy beach (details in Table 1, Figure 2A), used by local fishermen to berth boats when landing fish ( 01°55' 15.4” S; 59° 29' 58.5” W) . No adult otters were seen in the vicintity. No known den was nearby. According to the fishermen, the animal was relocated to a more visible location in hope of being rescued by any member of the group. However, the cub remained alone, vocalizing and appearing to be in pain. After 4 hours, the animal was taken to CPPMQA (Centro de Preservação e Pesquisa de Mamíferos e Quelônios Aquáticos) of Eletrobras Amazonas GT at Balbina village to receive neonatal care.
The animal was infested with fleas and, based on the loss of epidermal elasticity and low capillary perfusion time, had a moderate degree of dehydration (8 - 9%). The animal had difficulty urinating and defecating, only achieving this when stimulated in ways that mimicked the mother's natural hygienic care behavior. On January 8th the cub began to show abdominal distension. It showed signs of pain, was no longer able to urinate and rejected offered food. It died the same day. A necropsy revealed a distended bladder, occupying one third of the abdominal cavity, which probably resulted from a form of uremia linked to pneumonia. The pneumonia recorded in the necropsy was probably due to the animal's abandonment.
The second case reported here was also recorded in the Balbina hydroelectric reservoir (see Figure 1) where several studies have been carried out in order to determine the impact of hydroelectric lakes on giant otter populations (Rosas et al,. 2007; Cabral et al., 2010; Palmeirim et al., 2014; Rosas et al., 2015). As part of a radio telemetry project, at 02:30 on February 15th , 2014 a funnel-net trap was fixed on an active giant otter den with the aim of catching an otter for radio transmitter implantation (see Silveira et al., 2011 for tagging giant otters for telemetry studies). However, at 08:00 no giant otter had emerged from the den, and the only sound we could hear was the squeaks of a newborn cub (see Duplaix, 1980; Mumm and Knörnschild, 2014 for giant otter vocal repertoire). We concealed ourselves for a further three hours, during which time not a single adult was observed near the den. We then decided to open a trapdoor to investigate its interior, and using a flashlight, we saw a newborn cub completely alone inside the den (details in Table 1, Figure 2B). To avoid our presence causing undue stress, we then fully closed the trapdoor, leaving the cub within, and without having been touched by human hands, in the hope that the rest of the group would return to the cub. It is important to note that while the presence of humans in front of a giant otter den may cause the adults and sub adults to leave, they always return to the den once humans have gone. Such situations have been recorded at least five times in Balbina reservoir: in each case the cubs were moved to another den, but never abandoned them (Rosas et al., 2009; C.S. Ramalheira, personal observation).
Two days later on February 17th , we returned to the den to monitor developments, and to our surprise the newborn was still there, completely alone and dehydrated. We then realized that the cub had been left alone and took it to the Centro de Preservação e Pesquisa de Mamíferos e Quelônios Aquáticos (CPPMQA) at Balbina village. However, it did not survive long, dying on February 25th , 2014. Necropsy revealed pneumonia. However, we do not know whether this was the primary cause of mortality, or resulted from aspiration, thermal shock or other causes.
|
|
||||||||
| Table 1:Number of reported cases, date (month, day, year), sex, weight (g), total length (cm), estimated age (according to Bozzetti et al. 2015), and status of the individuals abandoned | ||||||||
|
|
||||||||
| Case | Date | Species | Sex | Weight | Total Length | Estimated Age | Stage | Observation |
| 1 | 01/03/1996 | P. brasiliensis | male | 620 | 46 | 2 weeks | newborn | eyes unopened |
| 2 | 02/15/2014 | P. brasiliensis | male | 1.050 | 52 | 3 weeks | newborn | eyes unopened |
| 3 | 10/02/2015 | P. brasiliensis | male | --- | 45 | 2 weeks | newborn | eyes unopened |
| 4 | 11/17/2010 | L. longicaudis | male | 1.700 | 75 | --- | cub | eyes opened |
|
|
||||||||
The third record occurred on the middle Rio Içana (São Gabriel da Cachoeira municipality, Amazonas State, Brazil), Rio Negro basin (see Figure 1), where lakes and streams were visited between October and November 2015 during field research on how landscape characteristics affect otter distribution (Pimenta, 2016). At 10:30 on October 28 th 2015, while sampling the Ttdiziali Stream (01°44'75" N; 068°42'96” W), we observed the body of a newborn giant otter cub trapped in the roots of a flooded tree trunk (details in Table 1, Figure 2C). It showed no signs of having received aggression, nor did it have any visible abnormalities. The animal had died recently, as decomposition was not advanced, and no scavenger bites were apparent, but it already showed symptoms of rigor mortis ( which usually peaks about twelve hours after death, so the animal may have died around 10 p.m. the day before) .
Due to logistic limitations, it was not possible to perform a necropsy nor could the animal, or its tissues, be collected. However, observation of the immediately surrounding area leads us to propose that the cause of death was drowning. We found a flooded den some 100m from the body. It was surrounded with giant otter pawprints, indicating recent use. It is considered relevant that at dawn previous night as our discovery of the drowned cub, there had been great storm causing a rapid and substantial rise in river level, which was almost certainly responsible for the observed den flooding.
According to Rosas et al. (2009), newborn cubs remain in the den during the day with a babysitter around 50% of the time. The babysitter is usually a single adult from the group, but not a parent (alloparental care). However, for the other 50% of the time newborn animals are left alone in the den during the day. It is possible that, in this third case, the cub had been left alone just for a moment and fell into the water by itself, as reported elsewhere by Rosas et al. (2008). Nevertheless, the newborn seems to be abandoned in the den during a storm which occurred overnight, a time when the group is generally together and also when nocturnal predators are active. The absence of adults during the night suggests that the cubs had not been momentarily left alone, but has been genuinely abandoned by the group.
The fourth record reported here is of a Lontra longicaudis cub (details in Table 1, Figure 2D). It was found by agents from the Uatumã Sustainable Development Reserve conservation team (see Figure 1), downstream of the Balbina dam in the northeastern Amazonas State, Brazil (IDESAM, 2008). On the morning of 17th November 2010, the animal was seen alone and vocalizing throughout the day on the shore of Lake Calabar (02°15'218” S; 058°56'30.6” W). The individual was monitored in situ by agents. After one day and one night of waiting, without success, for the return of adults the cub was taken to CPPMQA. It arrived on the morning of the 19th with signs of blindness, discoordination and pneumonia, and started to receive appropriate treatment for recovery.
After one week of treatment it no longer showed symptoms of pneumonia. However, on the morning of 2nd January 2011 the animal began to appear lethargic, and was found dead at 14:00 of the same day. A necropsy indicated the cause of death had been congestive pneumonia and cerebral lesions caused by hydrocephaly . The data from the necropsy of this individual, which indicated the animal had hydrocephaly, raises the possibility that in this specific case the animal was left behind due to abnormalities it possessed (which would have prevented it reaching adulthood).
According to Duplaix (1980), giant otter cubs do not leave the den until they are three weeks old (by which time their eyes are open). At one month, with the eyes now opened, they are able to swim, and with 6 weeks cubs regularly follow their parents out of the den and play around the den's entrance with supervision from parents or sub-adult siblings, but do not begin hunting and travelling with the group until they are 3-4 months old (Carter and Rosas, 1997).
In this context, it is notable that all giant otters abandonment records reported here were newborn cubs, which certainly would not have survived without parental/alloparental care. In 15 years of constant monitoring giant otters on Balbina Lake, not a single incident has been recorded of juveniles or individuals from the pre-dispersal age cohorts being abandoned. This scenario has also been found for the European otter (Poledník et al., 2011), and fits with predictions of optimal parental investment theory (Trivers, 1972; Low, 1978; Lycett et al., 1998; Chalfoun and Martin, 2010), and also with the notion that young will be most vulnerable to accidents (Rosas et al., 2009).
It is possible that some of the cases reported here represent a disordered behavioral response by the otters caused by anthropic presence (Lima and Marmontel, 2011). Nevertheless, offspring abandonment has been shown as a natural behavior in many mammal species and tends to occur due to the presence of anomalies, congenital diseases or a larger number of cubs in a litter than can be supported under current conditions. Doing so conserves energy that can then be allocated to the care of other cubs, or to allocate energy for a future pregnancy that might bring greater reproductive success (see Ross et al., 1959; Low, 1978; Eloff, 1980; Tait, 1980; Eowle and Dodge, 1989; Laurenson, 1994; Maniscalco et al., 2008; White, 2008 for mammals; and Burger, 1982 for a case in birds).
Giant otters’ reproductive peak usually occurs at the receding water season (Duplaix, 1980; Laidler, 1984; Evangelista and Rosas, 2011; Rosas et al., 2007), probably as an adaptive behavior to waters dynamic in Amazon, which would facilitate the capture of fish at the end of gestation and the onset lactation to restore energetic and nutritional demands of adult females postpartum (Rosas et al., 2007). In this context, we suggest that those cubs of P. brasiliensis found in the Balbina reservoir (case 1 and 2), born during a period which, based on data from the last 10 years (Bozzetti et al., 2015) is outside the species regional birth peak, could have been abandoned by the group as a way to save energy in a period of limited food resources. Similarly, living in a place with of lack resources due to nutrient poverty such as the upper Rio Negro, it is possible that the cub found at Içana (case 3) was left behind in an emergency situation so as to favor the healthier cubs of the litter, since providing milk or a large offspring may require an intense energetic demand that is difficult to supply in such a challenging environment.
The hydrocephaly detected in the L. longicaudis cub (case 4) supports the hypothesis of leaving cubs behind due to illness (see Simpson et al. 2008 for a parallel case in European otter). As for the cases which necropsy revealed pneumonia, this may have been caused by low immune capacity due to nutritional deficiency and hypothermia, which draws attention to the costly investment required by adults to ensure otter cub care and successfully raise offspring. The fact that all individuals that were left behind were male also raises the issue of the role of females in mammal populations. In cases of cub abandonment through lack of resources to support a large offspring, it is possible that there would be a bias in abandonments toward males, since females are responsible for generating and nursing the cubs, so playing a key role in population persistence (Cameron, 2004; West et al., 2005).
The cases reported here of abandonment of still-dependent P. brasiliensis and L. longicaudis cubs clearly demonstrate that this behavior occurs in at least two species of the sub-family Lutrinae, in addition to those cases already recorded for the better-studied European otter (Kruuk et al., 1991; Poledník et al., 2011). More attention should be given to studies in the areas of distribution of both species so that we can accumulate evidence that will help us to understand the circumstances in which this behavior is manifested and its effects on population dynamics of both species, especially when the stress-sensitivity of otter is considered and the potential this has for promoting cub abandonment (Mumm et al., 2014).
Acknowledgements: We thank the Fundação Grupo Boticário de Proteção à Natureza, Reserva Biológica do Uatumã (ReBio Uatumã/ICMBio), Amazonas Energia S.A., Philadelphia Zoo/USA, Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq, Associação dos Amigos para a Proteção do Peixe-boi da Amazônia – AMPA (Proj. Mamíferos Aquáticos da Amazônia sponsored by Petrobras Socioambiental), The Rufford Foundation, Idea Wild and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for logistical and/or financial support; the Fundação Nacional do Índio (FUNAI), Federação das Organizações Indígenas do Rio Negro (FOIRN), and Organização Indígena da Bacia do Içana (OIBI) for facilitating the research in Baniwa territory. This is contribution number 14 from the Amazon Mammal Research Group, INPA, Manaus, Brazil. .
REFERENCES
Bozzetti, B. F., Cabral, M.M.M., Rosas, F.C.W. (2015).
Cub biometry, litter size and reproductive period of giant otters ( Pteronura brasiliensis ) at the Balbina
Hydroelectric Reservoir, Amazonas, Brazil. Latin American Journal of Aquatic Mammals 10 : 115-121.
Burger, J. (1982) The role of reproductive success in colony-site selection
and abandonment in Black Skimmers ( Rynchops niger ). The Auk 99 : 109-115.
Cabral, M.M.M., Zuanon, J.A.S., de Mattos, G.E., Rosas, F.C.W. (2010). Feeding habits of giant otters
Pteronura brasiliensis (Carnivora: Mustelidae) in the Balbina hydroelectric reservoir, Central Brazilian Amazon.
Zoologia 27 : 47–53.
Cabral, M.M.M., Ribas, C., Rosas, F.C.W. (2015).
A case study of artificial feeding of an unweaned giant otter ( Pteronura brasiliensis ) cub. Latin American
Journal of Aquatic Mammals 10 : 159-162.
Cameron, E.Z. (2004). Facultative adjustment of mammalian sex ratios in support of the Trivers-Willard hypothesis: evidence for a mechanism. Biological Sciences 271 : 1723-1728.
Carter, S.K., Rosas, F.C.W. (1997). Biology and conservation of the giant otter Pteronura brasiliensis . Mammal Review 27 :1-26.
Chalfoun, A.D., Martin, T.E. (2010). Parental investment decisions in response
to ambient nest-predation risk versus actual predation on the prior nest. The Condor 112 : 701-710.
Charnov, E.L., Ernest, S.M. (2006). The offspring-size/clutch-size trade-off in mammals. The American Naturalist 167 : 578-582.
Doolan, S.P., Macdonald, D.W. (1997). Breeding and juvenile survival among slender-tailed meerkats
( Suricatu suricatta ) in the south-western Kalahari: ecological and social influences. Journal of Zoology
242 : 309-327.
Duplaix, N. (1980). Observations on the ecology and behavior of the giant river otter Pteronura brasiliensis in Suriname. Revue Ecologique (Terre Vie) 34 : 495-620.
Duplaix, N., Waldemarin, H.F., Groenendijk, J., Evangelista, E., Munis, M., Valesco, M .,
Botello, J.C. (2008) Pteronura brasiliensis . The IUCN Red List of Threatened Species . Version 2014.2. <www.iucnredlist.org>. Accessed on 30 Sept. 2014 .
Eloff, F.C. (1980). Cub mortality in the Kalahari lion Panthera leo vernayi (Roberts, 1948). Koedoe 23 : 163-170.
Elowe, K.D., Dodge, W.E. (1989). Factors affecting black bear reproductive success and cub survival. The Journal of Wildlife Management 53 : 962-968.
Evangelista, E., Rosas, F.C.W. (2011). Breeding behavior of giant otters ( Pteronura brasiliensis ) in the Xixuaú Reserve, Roraima, Brazil. IUCN Otter Specialist Group Bulletin 28A : 5-10.
Hauer, S., Ansorge, H., Zinke, O. (2002). Reproductive performance of otters Lutra lutra (Linnaeus, 1758) in Eastern Germany: low reproduction in a long-term strategy. Biological Journal of the Linnean Society 77 : 329-340.
IBAMA. (1997). Plano de Manejo Fase I: Reserva Biológica do Uatumã. Eletronorte/IBAMA. Brasília/DF/Brasil. <http://www.icmbio.gov.br> (downloaded 23.11.16).
IDESAM. (2008). Plano de Gestão da Reserva de Desenvolvimento Sustentável do Uatumã. Instituto de Conservação e Desenvolvimento Sustentável do Amazonas 1 : 1-336.
Jefferies D. J. (1987). The effects of angling interests on otters, with particular reference to disturbance. [In: Angling and Wildlife in Freshwaters, ITE Symposium No. 19. P. S. Mailland and K. Turner, eds]. Institute of Terrestrial Ecology, Grange-over-Sands: 23–30.
Klug, H. and M.B. Bonsall (2007) When to care for, abandon or eat your offspring: the evolution of parental care and filial cannibalism. American Naturalist , 170 : 886-901.
Kruuk, H., Conroy, J.W.H., Moorhouse, A. (1991). Recruitment to a population of otters ( Lutra lutra ) in Shetland, in relation to fish abundance. Journal of Applied Ecology 28 : 95-101
Laidler, P.E. (1984). The Behavioural Ecology of the Giant Otter in Guyana. Ph.D Thesis, University of Cambridge, 296 pages.
Larivière, S. (1999). Lontra longicaudis. Mammalian Species 609 : 1-5.
Laurenson, M.K. (1994). High juvenile mortality in cheetahs ( Acinonyx jubatus ) and its consequences for maternal care. Journal of Zoology 234 : 387-408.
Leuchtenberger, C., Magnusson, W.E., Mourão, G. (2015). Territoriality of giant otter groups in an area with seasonal flooding. Plos One . DOI: 10.1371/journal.pone.0126073
Lima, D.S., Marmontel, M. (2011).Return to the wild and reintegration of a giant river otter ( Pteronura brasiliensis ) cub to its family group in Amanã Sustainable Development Reserve, Brazilian Amazon. Latin American Journal of Aquatic Mammals 9 : 164-167.
Low, B.S. (1978). Environmental uncertainty and the parental strategies of marsupials and placentals. The American Naturalist 112 : 197-213.
Lycett, J.E., Henzi, S.P., Barrett, L. (1998). Maternal investment in mountain baboons and the hypothesis of reduced care. Behavioral Ecology and Sociobiology 42 : 49-56.
Malcolm, J.R., Marten, K. (1982). Natural selection and the communal rearing of pups in African wild dogs ( Lycaon pictus ). Behavioral Ecology and Sociobiology 10 : 1-13.
Maniscalco, J.M., Calkins, D.G., Parker, P., Atkinson, S. (2008). Causes and extent of natural mortality among Steller sea lion ( Eumetopias jubatus ) pups. Aquatic Mammals 34 : 277-287.
McTurk, D., Spelman, L. (2005). Hand-rearing and rehabilitation of orphaned wild giant otters, Pteronura brasiliensis, on the Rupunni River, Guyana, South America. Zoo Biology 24 : 153-167.
Mourão, G., Carvalho, L. (2001). Cannibalism among Giant Otter ( Pteronura brasiliensis ). Mammalia , 65 : 225-227.
Mumm, C.A.S., Knörnschild, M. (2014). The vocal repertoire of adult and neonate Giant Otters ( Pteronura brasiliensis ). PLoS One . DOI: 10.1371/journal.pone.0112562.
Mumm, C.A.S, Urrutia, M.C., Knörnschild, M. (2014). Vocal individuality in cohesion calls of giant otters, Pteronura brasiliensis. Animal Behaviour 88 : 243-252
Packer, C., Pusey, A.E. (1983). Adaptations of female lions to infanticide by incoming males. The American Naturalist 121 : 716-728.
Palmeirim, A.F., Peres, C.A., Rosas, F.C.W. (2014). Giant otter population responses to habitat expansion and degradation induced by a mega hydroelectric dam. Biological Conservation 174 : 30-38.
Pimenta, N.C. (2016). O Retorno das Ariranhas à Paisagem Baniwa. Master Thesis, Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Brazil.
Poledník, L., Poledníková, K., Vetrovcová, J., Hlavác, V., Beran, V. (2011). Causes of deaths of Lutra lutra in the Czech Republic (Carnivora: Mustelidae). Lynx 42 : 145-157.
Pontier, D., Gaillard, J.M., Allainé, D. (1993). Maternal investment per offspring and demographic tactics in placental mammals. Oikos 66 : 424-430.
Rosas, F.C.W., de Mattos, G.E. (2003). Natural deaths of giant otters ( Pteronura brasiliensis ) in Balbina hydroelectric lake, Amazonas, Brazil. IUCN Otter Specialist Group Bulletin 20 : 62-64.
Rosas, F.C.W, de Mattos, G.E., Cabral, M.M.M. (2007). The use of hydroelectric lakes by giant otters Pteronura brasiliensis : Balbina Lake in central Amazonia, Brazil. Oryx 41 : 520-524.
Rosas, F.C.W., Mendes Cabral, M.M. and Mattos, G.E. de (2008 ). Predation or Scavenging of Giant Otter ( Pteronura brasiliensis ) Cubs by Lizards? IUCN Otter Spec. Group Bull . 25 (1): 100 - 103
Rosas, F.C.W., Cabral, M.M.M., de Mattos, G.E., Silva, R.E. (2009). Parental and alloparental care of giant otters ( Pteronura brasiliensis ) (Carnivora, Mustelidae) in Balbina hydroelectric lake, Amazonas, Brazil. Sociobiology 54 : 919-924.
Rosas, F.C.W., Ramalheira, C.S., Bozzetti, B.F., Palmeirim, A.F., Cruz, A.D., Pathek, D.B., Cabral, M.M.M. (2015). Sleeping sites used by giant otters ( Pteronura brasiliensis ) in the Balbina hydroelectric reservoir, Central Brazilian Amazon. Aquatic Mammals , 41 : 143-148.
Ross, S., Denenberg, V.H., Frommer, G.P., Sawin, P.B. (1959). Genetic, physiological and behavioral background of reproduction in the Rabbit V. nonretrieving of neonates. Journal of Mammalogy 40 : 91-96.
Silveira, L., Furtado, M.M., Rosas, F.C.W., Silva, L.C., Cabral, M.M.M., Tôrres, N.M., Sollmann, R., Kouba, A., Jácomo, A.T. (2011). Tagging giant otters ( Pteronura brasiliensis ) (Carnivora, Mustelidae) for radio telemetry studies. Aquatic Mammals 37 : 208-212.
Simpson, V.R., Hargreaves, J., Birtles, R.J., Marsden, H., Williams, D.L. (2008). Tyzzer's disease in an Eurasian otter ( Lutra lutra ) in Scotland. Veterinary Record: Journal of the British Veterinary Association 163 : 539-543.
Staib, E., Schenck, C. (1994). Giant otters and ecotourism in Peru. IUCN Otter Specialist Group Bulletin 9 : 7-8.
Staib, E. (2005). Eco-etología del lobo del rio ( Pteronura brasiliensis ) en el Sureste del Perú. Spanish translation of German Ph.D. Thesis. Ayuda para Vida Silvestre Amenazada - Frankfurt Zoological Society. Lima, Peru.
Tait, D.E. (1980). Abandonment as a reproductive tactic- the example of grizzly bears. The American Naturalist 115 : 800-808.
Thompson, K.V., Baker, A.J., Baker, A.M. (2010). Parental care and behavioral development in captive mammals. In: Kleiman, D.G., Thompson, K.V., Baer, C.K. (Eds.) Wild mammals in captivity: principles and techniques. University of Chicago Press, Chicago, pp 367-385.
Trivers, R. (1972). Parental investment and sexual selection. Harvard University Press, Chicago. pp. 179.
West, A.S., Shuker, D.M., Sheldon, B.C. (2005). Sex-ratio adjustment when relatives interact: a test of constraints on adaptation. Evolution 59 : 1211-1228.
White, P.P. (2008). Maternal response to neonatal sibling conflict in the spotted hyena, Crocuta crocuta . Behavioral Ecology and Sociobiology 62 : 353-361.
Résumé : Seul à la Maison: Enregistrements d ’Abandon d’Individus toujours Dependents chez les Loutres Géantes (Pteronura brasiliensis) et les Loutres à Longue Queue (Lontra longicaudis) en Amazonie Brésilienne
En dépit de l'augmentation des études menées sur les loutres, la compréhension de certain comportement reste difficile, particulièrement ceux qui sont rarement observés. Cette étude présente trois cas d'abandon de petits par Pteronura brasiliensis, et un cas pour Lontra longicaudis. Les trois cas d'abandon observés pour la loutre géante ont eu lieu dans des habitats pauvres en nourriture. Le cas d'abandon pour la loutre à longue queue semble être lié à un mauvais état de santé du petit. Les rapports actuels, ainsi que les futurs rapports qui sont encouragés, peuvent être important quant au développement de plans de conservation pour les trois espèces de loutres puisque les morts naturelles peuvent avoir des implications dans la dynamique des populations. De tels rapports pourraient également contribuer à la compréhension des circonstances entourant le comportement d'abandon.
Revenez au dessus
Resumen: En Casa Solo: Registros de Abandono de Individuos a ún Dependientes de Nutrias Gigantes (Pteronura brasiliensis) y Nutrias Neotropicales (Lontra longicaudis), en el Amazonas Brasilero
A pesar del incremento en estudios sobre Lutrinae, el conocimiento sobre algunos comportamientos, especialmente
aquellos raramente vistos, sigue siendo limitado. Este estudio presenta tres casos de abandono de crías por
Pteronura brasiliensis , y uno por Lontra longicaudis . Los tres casos de abandono de nutria
gigante ocurrieron en hábitats pobres en nutrientes. El caso de nutria neotropical puede estar relacionado
con enfermedad de la cría. Estos reportes, y cualquier reporte futuro que pudiera surgir motivado por este
trabajo, podrían ser importantes al desarrollar planes de conservación para estas especies, ya que las
muertes naturales pueden tener implicancias para la dinámica poblacional. Tales reportes también
podrían contribuir a entender las circunstancias que rodean al comportamiento de abandono.
Vuelva a la tapa